- Ground-breaking British technology will initially provide hundreds of thousands of fast, accurate, low-cost COVID-19 tests under new agreement with the UK Government, with the potential to build to millions of tests per month
- The “LamPORE COVID-19” assay provides precise detection of SARS-CoV-2, the virus that causes COVID-19, using Oxford Nanopore’s DNA/RNA sequencing technology.
- LamPORE is fully scalable and designed to enable both high-volume screening and rapid, local testing. Results can be obtained in under two hours.
- In addition, the “LamPORE Respiratory Panel”, currently in development, is designed to detect multiple viruses in a single sample; not only SARS-CoV-2 but also the most common winter respiratory viruses including Influenza A and B and RSV.
Monday 3rd August, Oxford, UK: Oxford Nanopore today announces an agreement with the UK’s Department of Health and Social Care, to roll out its novel LamPORE test. This will support the UK’s efforts to manage the continued reduction of COVID-19 and containment of new cases, now and through the winter cold and flu season.
Under the agreement, an initial 450k LamPORE SARS-CoV-2 tests will be made available for use by a number of NHS testing laboratories. As well as providing a large number of tests for existing labs, the programme will help the UK to understand the different use cases for the technology, for example the potential asymptomatic screening of frontline staff.
Because of its scalability, LamPORE has the potential to provide both:
Large-scale screening to detect the virus in broader populations. Individuals being tested may be presymptomatic or asymptomatic, eg:
- Regular screening of frontline workforces
- Broad screening of a community/population in the event of an outbreak
- Screening at the point of congregation eg transport hubs
Rapid, focused, localised analysis, eg:
- In-hospital testing to protect staff and patients and enable operational capacity
- Care homes, education and elsewhere in the community health and care sector
- Leisure, culture or businesses
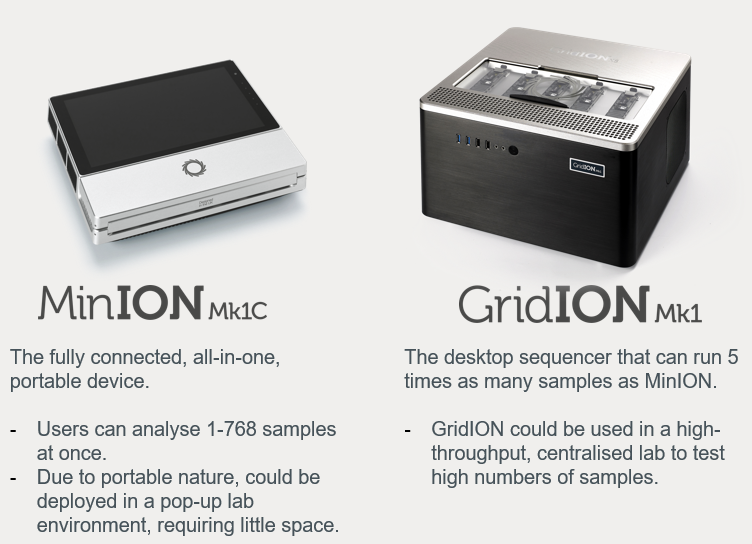
LamPORE is designed to be deployed on Oxford Nanopore’s desktop device (GridION) or palm-sized device (MinION Mk1C), providing the capacity of processing up to 15,000 samples a day or 2,000 samples a day respectively. It is well suited to use in a central laboratory for high-throughput sample processing, or near-community ‘pop-up lab’. LamPORE results can be generated in under two hours. The approach of having testing centres available in more locations combined with this speed supports rapid turnaround of results. Fast results can help precise isolation and therefore supports public health strategies to prevent onwards transmission of the virus.
LamPORE Respiratory Panel: a multiple-pathogen test
In addition to a test for SARS-CoV-2, the virus that causes COVID-19, Oxford Nanopore is currently developing LamPORE to test for multiple pathogens within a single sample, including influenza A (H1N1 and H3N2), influenza B, respiratory syncytial virus (RSV) and SARS-CoV-2. This is intended to allow healthcare professionals to distinguish between these infections, better manage expected winter pressures on the NHS and guide public health and clinical management of these diseases at a time of traditionally heightened pressure on health services. It also supports a goal of understanding dynamics between these viruses in the UK population.
Gordon Sanghera, CEO of Oxford Nanopore, said:
“We are honoured to be playing a part in fighting COVID-19 in the UK, and preparing the country for the winter virus season. Ever since we founded Oxford Nanopore, our mission has been to create disruptive, high- performance technology that has a profound, positive impact on society. LamPORE has the potential to deliver a highly effective and, crucially, accessible global testing solution, not only for COVID-19 but for a range of other pathogens. We are delighted to be working with the UK government to support and empower our communities to effectively manage testing at a national and localised level.”
Health Secretary Matt Hancock said:
“Oxford Nanopore’s new rapid LamPORE tests will benefit thousands of people with fast and accurate test results, removing uncertainty and breaking chains of transmission quickly and safely.
“I am hugely grateful for the fantastic work Oxford Nanopore have done to push forward this important innovation in coronavirus testing.”
About LamPORE: a new generation of testing technology
LamPORE detects active infection, providing a complementary testing solution to antibody detection, which is currently only able to indicate a previous infection.
- LamPORE is a precise, rapid, low-cost and highly scalable assay for the detection of SARS-CoV-2, the virus that causes COVID-19.
- It is designed to test saliva and swab RNA samples, whether gathered from people who are showing symptoms of COVID, or those who do not have symptoms.
- LamPORE is designed to be deployed, at scale, for a comparable cost per test as standard RT-PCR testing. However, unlike RT-PCR the analysis is not performed in duplicate/triplicate, thus reducing cost of double/triple-testing and expanding capacity.
- The LamPORE assay is performed on Oxford Nanopores devices, which are highly affordable and capable of performing thousands of tests per day, per device.
- LamPORE includes an internal control. This allows the user to identify that a test is invalid if the sample has not been gathered effectively, which can be a source of ‘false negative’ results.
- LamPORE uses Oxford Nanopore’s sequencing technology to precisely identify amplified sections of the SARS-CoV-2 virus, after it has been targeted and amplified using RT-LAMP. This method of detection can differentiate between actual SARS-CoV2 presence and errors that can occur during amplification, which can be a source of ‘false positive’ results.
- Furthermore, as LamPORE does not rely on the same components used in existing RT-PCR tests, it offers the potential to ease pressure on current supply chains and expand testing access.
LamPORE is the first assay developed by Oxford Nanopore for diagnostic use. Oxford Nanopore is currently in the process of CE marking the device for diagnostic use. The company will also be pursuing regulatory clearance in other jurisdictions.
Oxford Nanopore was spun out of the University of Oxford, and has since developed and brought to market a new generation of tools for biological analysis. The Company opened up a new, high-tech manufacturing facility in Oxford in 2019 to support rapid-scaleup of production.
LamPORE is not offered as a testing service to the UK public. If you are a laboratory or another institution that is scaling up or planning operations, you can get in touch with us here.